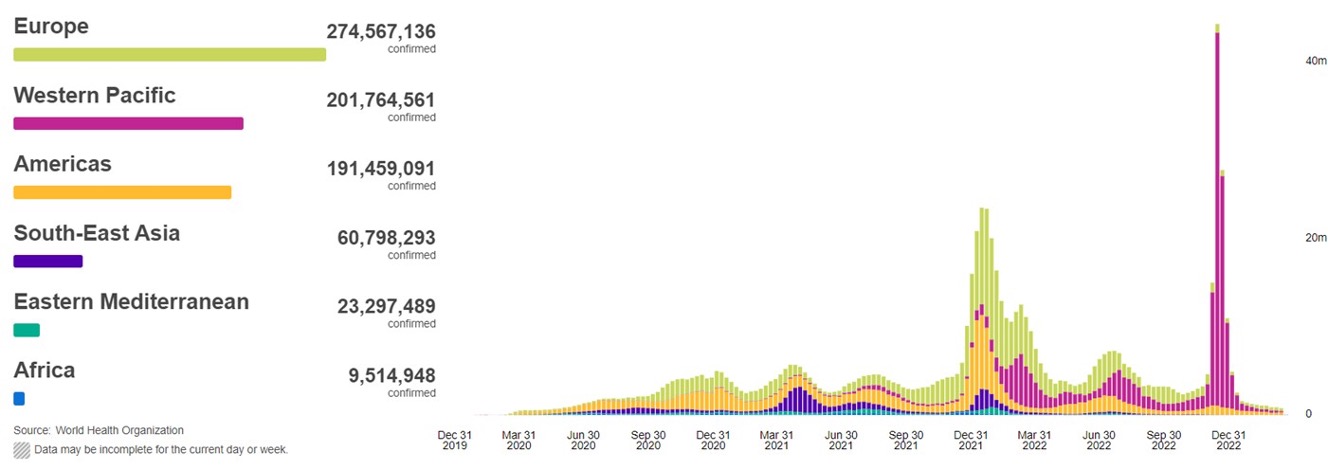
SARS-CoV-2 virus is the causative agent of the continuing epidemic, which has resulted in over six million deaths worldwide, as well as considerable impairment of general health amongst survivors due to numerous symptoms sometimes referred to as 'long COVID'. The trimeric spike glycoprotein, the predominant surface protein of SARS-CoV-2 and the primary target of neutralizing antibodies, is the sole immunogen in most COVID-19 vaccines. The principal target of the neutralizing response is the Receptor Binding Domain (RBD), and serum titers against the RBD correlate strongly with neutralization titers. Many viral variants of concern (VOC) have appeared in recent months. Current VOCs are derived from the BA.1 (Omicron) lineage, and these are poorly neutralized by most existing, clinically approved antibodies.
Current Vaccines
Researchers around the world are continuing to develop improved COVID-19 vaccines. These vaccine candidates are designed to enhance the body’s immune system to safely recognize and block the virus that causes COVID-19 disease.
Several different types of vaccine candidates have been developed and licensed for human use including inactivated virus vaccines, adenoviral vectored vaccines, protein subunit vaccines, and mRNA vaccines. Protein subunit vaccines use harmless fragments of proteins such as the spike protein that mimic the COVID-19 virus to safely generate an immune response. Viral vector vaccines use a safe virus such as an adenovirus or a MVA that cannot cause disease or replicate inside our bodies but serve as a platform to produce the Spike surface protein of SARS-CoV-2 to generate an immune response. mRNA vaccines are a new approach that uses genetically engineered, appropriately formulated mRNA that is injected into the body, which is then taken up by cells, leading to production of the SARS-CoV-2 Spike surface protein, thus generating immune responses able to protect against COVID-19 disease.
Lead Candidates
Myncov1003, a clinical stage, adjuvanted, protein subunit vaccine, is based on an engineered Receptor Binding Domain (RBD) of the Spike surface protein of the Corona virus. Myncov1003 is a thermotolerant vaccine candidate which can be stored at room temperature (up to 40oC) for about a month without any significant drop in potency. Sera elicited by Myncov1003 can neutralize a broad spectrum of VOCs of SARs-CoV-2, starting from the original Wuhan strain to the recent Omicron VOCs. Myncov1003 is now ready for human clinical development.
Myncov1006, a pre-clinical stage pipeline candidate, is an engineered and potent derivative of the Spike protein which includes the RBD as well as conserved regions of the Spike protein. Myncov1006 elicits strong protection in animal challenge models such as hAce2 transgenic mice and hamsters and is able to protect against challenge with various strains of SARS-CoV-2 including Omicron VOCs.
Myncov1003 and Myncov1006 are produced in high expressing, stable CHO cell lines that are compatible with large scale manufacturing.